Isoleucyl-tRNA Synthetase 2 (IARS2) Promotes Pancreatic Ductal Adenocarcinoma (PDAC)
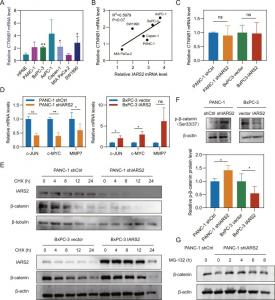
(A) Relative CTNNB1 mRNA level in normal and pancreatic adenocarcinoma cell lines. (B) The correlation between relative IARS2 mRNA transcription level and CTNNB1 mRNA transcription level. (C) CTNNB1 mRNA level remained unchanged after IARS2 knockdown or o
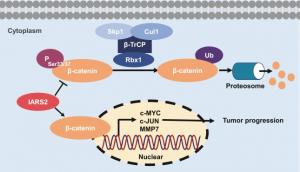
IARS2 facilitates PDAC proliferation and metastasis by stabilizing β-catenin and activating the WNT/β-catenin pathway.
CHINA, March 18, 2025 /EINPresswire.com/ -- Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related mortality worldwide. Current treatment modalities are few or ineffective due to PDAC’s heterogeneous molecular patterns, which cause it to respond variably to the same therapy. Understanding the molecular taxonomy of PDAC phenotypes may open avenues for therapeutic interventions.
A recent study published in the Genes & Diseases journal by researchers at Shanghai Jiao Tong University School of Medicine implicates isoleucyl-tRNA synthetase 2 (IARS2) in promoting the proliferation and metastasis of PDAC.
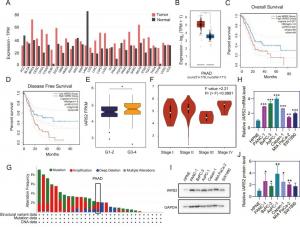
Isoleucyl-tRNA synthetase 2 (IARS2) has previously been shown to be dysregulated in various cancers. This study showed that IARS2 expression was higher in PDAC tissues and cell lines. Furthermore, high IARS2 expression was associated with a shorter overall survival and disease-free survival time and was significantly higher in grade 3–4 pancreatic cancer stages compared to grade 1–2. In vitro and in vivo experiments involving IARS2 knockdown and overexpression revealed that IARS2 promotes the proliferation, migration, and invasion of PDAC cells via upregulation of EMT transcription factors.
Transcriptomic analysis of the TCGA-PAAD cohort showed that IARS2 regulates biological processes. Gene set enrichment analysis (GSEA) showed that WNT signaling, cell cycle, p53, and apoptotic pathways were enriched in the high-IARS2 expression group of TCGA-PAAD. The gene expression-based stemness index (mRNAsi) was higher in patients with high IARS2 expression. The expression of cancer stemness markers, like CD44, MET, CD133, FUT4, ACVR1, mTOR, and KLF4 correlated positively with IARS2 expression, suggesting that IARS2 promotes metastasis via regulation of cancer cell stemness. Additionally, high IARS2 expression was associated with lower infiltration of CD8+ T cells, indicating that IARS2 promotes an immunosuppressive microenvironment.
This study also showed that IARS2 protects β-catenin from phosphorylation-dependent proteasome degradation by β-TrCP (F-box protein beta-transducin repeat containing protein). Further results established that IARS2 promotes pancreatic tumorigenesis via activation of the Wnt/ β-catenin pathway by stabilizing β-catenin.
In conclusion, this study showed that up-regulated IARS2 in PDAC tissues correlates with poor prognosis and that IARS2 facilitates PDAC proliferation and metastasis by stabilizing β-catenin and activating the WNT/β-catenin pathway. These results indicate that IARS2 may serve as an underlying prognostic marker and a potential therapeutic target for PDAC. However, this study has limitations and need more experiments to explore how IARS2 impacts β-catenin degradation and the specific interaction between IARS2 and GSK3β.
Reference
Title of the original paper - Isoleucyl-tRNA synthetase 2 promotes pancreatic ductal adenocarcinoma proliferation and metastasis by stabilizing β-catenin
Journal - Genes & Diseases
Genes & Diseases is a journal for molecular and translational medicine. The journal primarily focuses on publishing investigations on the molecular bases and experimental therapeutics of human diseases. Publication formats include full length research article, review article, short communication, correspondence, perspectives, commentary, views on news, and research watch.
DOI - https://doi.org/10.1016/j.gendis.2024.101382
Funding Information:
National Natural Science Foundation of China (No. 81870385, 81702740).
# # # # # #
Genes & Diseases publishes rigorously peer-reviewed and high quality original articles and authoritative reviews that focus on the molecular bases of human diseases. Emphasis is placed on hypothesis-driven, mechanistic studies relevant to pathogenesis and/or experimental therapeutics of human diseases. The journal has worldwide authorship, and a broad scope in basic and translational biomedical research of molecular biology, molecular genetics, and cell biology, including but not limited to cell proliferation and apoptosis, signal transduction, stem cell biology, developmental biology, gene regulation and epigenetics, cancer biology, immunity and infection, neuroscience, disease-specific animal models, gene and cell-based therapies, and regenerative medicine.
Scopus CiteScore: 7.3 | Impact Factor: 6.9
# # # # # #
More information: https://www.keaipublishing.com/en/journals/genes-and-diseases/
Editorial Board: https://www.keaipublishing.com/en/journals/genes-and-diseases/editorial-board/
All issues and articles in press are available online in ScienceDirect (https://www.sciencedirect.com/journal/genes-and-diseases).
Submissions to Genes & Disease may be made using Editorial Manager (https://www.editorialmanager.com/gendis/default.aspx ).
Print ISSN: 2352-4820
eISSN: 2352-3042
CN: 50-1221/R
Contact Us: editor@genesndiseases.com
X (formerly Twitter): @GenesNDiseases (https://x.com/GenesNDiseases)
Genes & Diseases Editorial Office
Genes & Diseases
+86 23 6571 4691
email us here
Visit us on social media:
Facebook
X
LinkedIn
Instagram
YouTube
Other
Distribution channels: Education, Healthcare & Pharmaceuticals Industry, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release