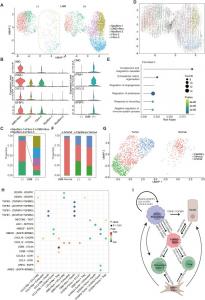
Transcriptional Landscape of PDAC Shows Distinct Cell Populations
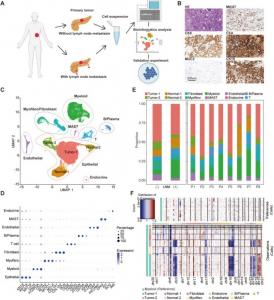
(A) Schematic workflow of sample collection for further single-cell RNA sequencing. (B) The hematoxylin and eosin staining and immunohistochemistry staining of tumor sample from P6. (C) UMAP plots of all cells from 8 PDAC samples colored by cell types. (D
Single-cell RNA sequencing (scRNA-seq) on a PDAC cohort identified distinct cell populations associated with tumor initiation and progression
CHINA, March 17, 2025 /EINPresswire.com/ -- Pancreatic cancer is one of the leading causes of cancer-related mortality, with pancreatic ductal adenocarcinoma (PDAC) accounting for 90% of all cases. As most PDAC cases are diagnosed at advanced stages, surgical interventions are ineffective, and consequently, lymph node metastasis manifests in 70% of PDAC patients. Moreover, since the number of genetic mutations giving rise to PDAC are low, there are fewer available targeted therapeutic modalities. Analysis of gene expression at the single-cell level may help understand the tumor dynamics of PDAC.
In a recent study published in the Genes & Diseases journal, researchers at Fudan University Shanghai Cancer Center and Shanghai Jiao Tong University School of Medicine performed single-cell RNA sequencing (scRNA-seq) on eight PDAC patients with varying lymph node metastasis (LNM) statuses to understand the transcriptional landscape regulating PDAC development and identify novel therapeutic targets.
scRNA-seq of LNM and non-LNM primary PDAC showed a heterogeneous cellular composition and identified four distinct cell populations: the MMP1+ & S100A2+ tumor cells, CCL2+ macrophages, and OMD+ fibroblasts. An integrated analysis comparing PDAC and normal pancreatic epithelial tissues revealed a pancreatic intraepithelial neoplasia (PanIN) subset with increased ONECUT2 (one cut domain family member 2) expression. This subset had a high MMP1 expression and is an intermediary between normal acinar cells and PDAC cells. Further results showed that MMP1 predicts unfavorable prognosis in PDAC patients and plays an important role in sustaining ductal identity in PDAC.
The S100A2+subset cells had increased expression of genes associated with tumor progression and poor prognosis. In vitro studies showed that S100A2 enhances the migratory capability of cancer cells while its knockdown led to decreased expression of EMT genes and cell-adhesion mediated drug resistance. Analysis of the immune cell subsets in the PDAC microenvironment showed a higher presence of pro-metastatic sub-populations within the immune cell milieu in PDAC tumors with LNM than in PDAC without LNM. This population was rich in CCL2+ macrophages, which play a role in EMT, immunosuppression, and indirect activation of cytotoxic CD8+ cells and are associated with poor disease-specific survival. Analysis of the stromal compartment revealed a subset of OMD+ fibroblasts that regulate tumorigenesis and metastasis by recruiting CCL2+ macrophages to create a pro-tumor environment.
In conclusion, this study identified OMD+ fibroblasts, CCL2+ macrophages, S100A2+, and MMP1+ tumor cells as key cell subsets that collectively contribute to a pro-tumor microenvironment conducive to LNM in PDAC and may serve as potential therapeutic targets.
Reference
Title of the original paper - The scRNA-sequencing landscape of pancreatic ductal adenocarcinoma revealed distinct cell populations associated with tumor initiation and progression
Journal: Genes & Diseases
Genes & Diseases is a journal for molecular and translational medicine. The journal primarily focuses on publishing investigations on the molecular bases and experimental therapeutics of human diseases. Publication formats include full length research article, review article, short communication, correspondence, perspectives, commentary, views on news, and research watch.
DOI: https://doi.org/10.1016/j.gendis.2024.101323
Funding Information:
National Natural Science Foundation of China (No. 82272095, 82072638 and 82002537).
Zhangjiang National Innovation Demonstration Zone (Shanghai, China) (No. ZJ2021-ZD-007)
The Biobank Program of Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine (Shanghai, China) (No. YBKB202217)
# # # # # #
Genes & Diseases publishes rigorously peer-reviewed and high quality original articles and authoritative reviews that focus on the molecular bases of human diseases. Emphasis is placed on hypothesis-driven, mechanistic studies relevant to pathogenesis and/or experimental therapeutics of human diseases. The journal has worldwide authorship, and a broad scope in basic and translational biomedical research of molecular biology, molecular genetics, and cell biology, including but not limited to cell proliferation and apoptosis, signal transduction, stem cell biology, developmental biology, gene regulation and epigenetics, cancer biology, immunity and infection, neuroscience, disease-specific animal models, gene and cell-based therapies, and regenerative medicine.
Scopus CiteScore: 7.3 | Impact Factor: 6.9
# # # # # #
More information: https://www.keaipublishing.com/en/journals/genes-and-diseases/
Editorial Board: https://www.keaipublishing.com/en/journals/genes-and-diseases/editorial-board/
All issues and articles in press are available online in ScienceDirect (https://www.sciencedirect.com/journal/genes-and-diseases).
Submissions to Genes & Disease may be made using Editorial Manager (https://www.editorialmanager.com/gendis/default.aspx ).
Print ISSN: 2352-4820
eISSN: 2352-3042
CN: 50-1221/R
Contact Us: editor@genesndiseases.com
X (formerly Twitter): @GenesNDiseases (https://x.com/GenesNDiseases)
Genes & Diseases Editorial Office
Genes & Diseases
+86 23 6571 4691
email us here
Visit us on social media:
Facebook
X
LinkedIn
Instagram
YouTube
Other
Distribution channels: Education, Healthcare & Pharmaceuticals Industry, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release