
HER2+ Non-Small Cell Lung Cancer Market is Predicted to Grow at a CAGR of 13.2% During the Study Period (2020–2034) | DelveInsight
The treatment landscape for HER2-positive NSCLC is rapidly evolving as research continues to reveal the genetic drivers of these tumors. Emerging therapies like Pyrotinib (Jiangsu HengRui Medicine), Zongertinib (Boehringer Ingelheim), and BAY2927088 (Bayer) are drawing significant attention for their potential in HER2-mutant NSCLC.
/EIN News/ -- New York, USA, Feb. 17, 2025 (GLOBE NEWSWIRE) -- HER2+ Non-Small Cell Lung Cancer Market is Predicted to Grow at a CAGR of 13.2% During the Study Period (2020–2034) | DelveInsight
The treatment landscape for HER2-positive NSCLC is rapidly evolving as research continues to reveal the genetic drivers of these tumors. Emerging therapies like Pyrotinib (Jiangsu HengRui Medicine), Zongertinib (Boehringer Ingelheim), and BAY2927088 (Bayer) are drawing significant attention for their potential in HER2-mutant NSCLC.
DelveInsight’s HER2+ Non-Small Cell Lung Cancer Market Insights report includes a comprehensive understanding of current treatment practices, emerging HER2+ NSCLC drugs, market share of individual therapies, and current and forecasted HER2+ NSCLC market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Key Takeaways from the HER2+ Non-Small Cell Lung Cancer Market Report
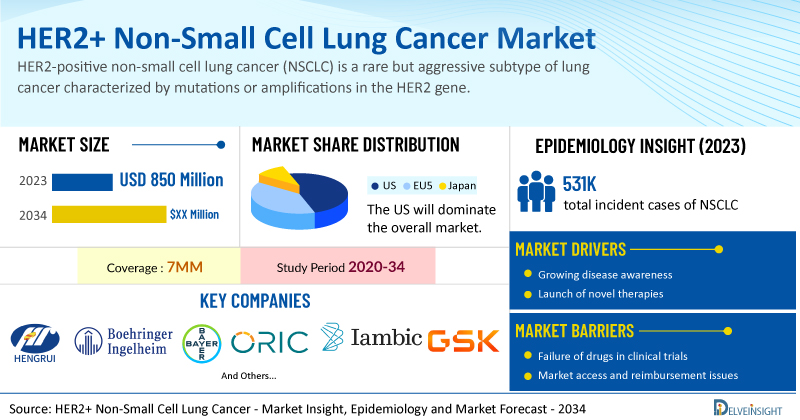
- According to DelveInsight’s analysis, the market size of HER2 NSCLC (Mutant, Overexpression, and Amplification) in the 7MM was USD 850 million in 2023.
- In 2023, among the therapies for HER2 NSCLC, the highest revenue was generated by Checkpoint inhibitors ± Chemotherapy, i.e., USD ~480 million in the US.
- NSCLC comprises approximately 80– 85% of all lung cancer diagnoses, with HER2 gene mutation found in approximately 1–4% of NSCLC cases. In the US, HER2 amplification and overexpression are observed in 2–5% and 2–30% of NSCLC cases, respectively.
- In 2023, the total incident cases of NSCLC in 7MM was ~531K, out of which the contribution of the US was ~38%.
- Prominent companies working in the domain of HER2+ NSCLC, including Jiangsu HengRui Medicine, Boehringer Ingelheim, Bayer, Forward Pharmaceuticals, ORIC Pharmaceuticals, Iambic Therapeutics, ArriVent BioPharma, Mersana Therapeutics, GSK, Nuvalent, and others, are actively working on innovative HER2+ NSCLC drugs. These novel HER2+ NSCLC therapies are anticipated to enter the HER2+ NSCLC market in the forecast period and are expected to change the market.
- Some of the key HER2+ NSCLC therapies in the pipeline include Pyrotinib, Zongertinib, BAY2927088, FWD1509, ORIC-114, IAM1363, Firmonertinib, XMT-2056, NVL-330, and others.
- In September 2024, AstraZeneca presented the first data of the ENHERTU monotherapy cohort for second or later-line treatment at WCLC from the Phase Ib clinical trial for HER2 positive nonsquamous NSCLC (DESTINY-Lung03), building on data from the DESTINY-Lung01 Phase II trial.
- In September 2024, Boehringer Ingelheim reported positive results from a Phase Ib primary analysis of Cohort 1 of the Beamion LUNG-1 trial evaluating zongertinib in pretreated patients with advanced NSCLC with activating HER2 mutations.
- In August 2024, Bayer announced that the first patient had been enrolled in the global Phase III SOHO-02 trial, an open-label, randomized, multicenter clinical trial, assessing the efficacy and safety of investigational agent BAY 2927088 as first-line therapy in patients with advanced NSCLC, whose tumors have activating HER2 mutations.
- In June 2024, Bayer presented the safety and clinical activity of BAY 2927088 from a Phase I/II trial expansion cohort in patients with HER2-mutant NSCLC at the 2024 ASCO annual meeting.
Discover which therapies are expected to grab the HER2+ NSCLC market share @ HER2+ Non-Small Cell Lung Cancer Market Report
HER2+ Non-Small Cell Lung Cancer Overview
HER2-positive non-small cell lung cancer (NSCLC) is a rare but aggressive subtype of lung cancer characterized by mutations or amplifications in the HER2 (human epidermal growth factor receptor 2) gene. These genetic alterations drive uncontrolled tumor growth and are found in approximately 2-4% of NSCLC cases, more commonly in non-smokers and younger patients. The exact cause of HER2 mutations in lung cancer remains unclear, but they are generally linked to genetic predisposition rather than environmental factors like smoking.
Symptoms of HER2+ NSCLC are similar to other lung cancers and may include persistent cough, shortness of breath, chest pain, unexplained weight loss, and fatigue. Diagnosis typically involves imaging techniques such as CT scans and PET scans, followed by a biopsy to confirm the presence of cancer cells. Molecular testing is crucial for detecting HER2 mutations or amplifications, helping to guide targeted therapy decisions. Due to its aggressive nature, HER2+ NSCLC often requires personalized treatment strategies, including targeted therapies such as HER2 inhibitors and antibody-drug conjugates (ADCs).
HER2+ Non-Small Cell Lung Cancer Epidemiology Segmentation
The HER2+ NSCLC epidemiology section provides insights into the historical and current HER2+ NSCLC patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The HER2+ NSCLC market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Incident Cases of NSCLC
- Gender-specific Cases of NSCLC
- Age-specific Cases of NSCLC
- Total Incident Cases of NSCLC by Histology
- Total Incident Cases of NSCLC by Stage
- Total Incident Cases of Advanced HER2 NSCLC (Mutant, Overexpression, and Amplification)
Download the report to understand which factors are driving HER2+ NSCLC epidemiology trends @ HER2+ Non-Small Cell Lung Cancer Epidemiological Insights
HER2+ Non-Small Cell Lung Cancer Treatment Market
HER2 alterations are key oncogenic drivers in NSCLC. In recent years, significant progress has been made in understanding HER2-driven disease and evaluating the effectiveness of various HER2-targeting therapies. Currently, platinum-based chemotherapy, with or without immunotherapy, remains the preferred first-line treatment for patients with advanced or metastatic HER2-mutant NSCLC. However, traditional treatments—including chemotherapy, immunotherapy, checkpoint inhibitors, and targeted cell therapy—have historically resulted in a median progression-free survival (PFS) of only about four months, highlighting the urgent need for more effective therapies. ENHERTU is the only FDA-approved drug used as the treatment approach for patients with HER2 NSCLC.
ENHERTU is a conjugate of a HER2-targeted antibody and topoisomerase inhibitor, approved for treating adult patients with unresectable or metastatic non-small cell lung cancer (NSCLC) whose tumors have activating HER2 (ERBB2) mutations, identified through an FDA-approved test, and who have previously undergone systemic therapy.
The FDA approved ENHERTU in August 2022 for adults with previously treated HER2-mutant metastatic NSCLC. This approval was granted under the FDA’s accelerated approval pathway based on the objective response rate (ORR) and duration of response (DoR). Ongoing approval may depend on confirming the clinical benefit through a subsequent trial. In August 2023, ENHERTU received approval from Japan’s Ministry of Health, Labour and Welfare (MHLW) for HER2-positive NSCLC, followed by European approval the next month. The company is now working on expanding ENHERTU’s use in the first-line treatment setting with a Phase III clinical trial, with topline results expected by 2025, leveraging its success in second-line treatment.
Learn more about the market of HER2+ NSCLC @ HER2+ Non-Small Cell Lung Cancer Treatment
HER2+ Non-Small Cell Lung Cancer Emerging Drugs and Companies
Some of the drugs in the pipeline include Pyrotinib (Jiangsu HengRui Medicine), Zongertinib (Boehringer Ingelheim), BAY2927088 (Bayer), and others.
Zongertinib (also known as BI 1810631) is an experimental oral HER2-specific TKI being developed as a potential treatment for HER2-mutated NSCLC. The drug received FDA Fast Track Designation in 2023, followed by Breakthrough Therapy Designation in 2024 from both the US FDA and China CDE for adults with advanced NSCLC that harbors activating HER2 mutations and has progressed after prior systemic therapy.
Currently, the company is assessing the drug in a Phase III clinical trial (Beamion LUNG-2) for first-line treatment in patients with unresectable, locally advanced, or metastatic non-squamous NSCLC with HER2 tyrosine kinase domain mutations. Additionally, the Phase I Beamion LUNG-1 trial is evaluating zongertinib as a monotherapy in NSCLC patients with HER2 mutations. In September 2024, the company announced positive findings from the Phase Ib primary analysis of Cohort 1 in the Beamion LUNG-1 trial.
BAY 2927088 is an oral small-molecule tyrosine kinase inhibitor designed to selectively target mutant HER2, including HER2 exon 20 insertions and point mutations, as well as EGFR while maintaining strong selectivity for mutant over wild-type EGFR. In February 2024, the drug was granted Breakthrough Therapy designation for treating adult patients with unresectable or metastatic NSCLC harboring activating HER2 (ERBB2) mutations who have previously undergone systemic therapy.
Currently, BAY 2927088 is being assessed in the Phase III SOHO-02 clinical trial as a first-line treatment for patients with locally advanced or metastatic NSCLC with HER2-activating mutations. As per the Q2 2024 update, the company plans to submit the drug for second-line HER2-mutated NSCLC approval in 2025.
The other pipeline therapies for HER2+ NSCLC include
- FWD1509: Forward Pharmaceuticals
- ORIC-114: ORIC Pharmaceuticals
- IAM1363: Iambic Therapeutics
- Firmonertinib: ArriVent BioPharma
- XMT-2056: Mersana Therapeutics/GSK
- NVL-330: Nuvalent
The anticipated launch of these emerging therapies are poised to transform the HER2+ NSCLC market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the HER2+ NSCLC market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about HER2+ NSCLC clinical trials, visit @ HER2+ Non-Small Cell Lung Cancer Treatment Drugs
HER2+ Non-Small Cell Lung Cancer Market Dynamics
The HER2+ NSCLC market dynamics are anticipated to change in the coming years. The market is primarily driven by the growing understanding of the genetic and molecular mechanisms underlying NSCLC, particularly the role of HER2 amplification or overexpression in a subset of patients. Advances in biomarker testing and personalized medicine have allowed for more accurate identification of HER2+ NSCLC patients, thereby increasing demand for targeted therapies. Additionally, the increasing prevalence of NSCLC, coupled with the unmet need for effective treatments in this subset of patients, has prompted significant investment in the development of HER2-targeted therapies, such as monoclonal antibodies and antibody-drug conjugates (ADCs).
Furthermore, many potential therapies are being investigated for the treatment of HER2+ NSCLC, and it is safe to predict that the treatment space will significantly impact the HER2+ NSCLC market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate is expected to drive the growth of the HER2+ NSCLC market in the 7MM.
However, several factors may impede the growth of the HER2+ NSCLC market. One of the primary challenges is the limited availability of effective targeted therapies, as HER2 amplification or mutation in NSCLC is less common than in other cancers, making it harder to identify appropriate patient populations. Additionally, the heterogeneity of HER2+ tumors and the lack of standardized testing for HER2 mutations complicate diagnosis and treatment selection. The high cost of innovative therapies, such as monoclonal antibodies or ADCs, also poses a financial burden on healthcare systems and patients.
Moreover, HER2+ NSCLC treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, the HER2+ NSCLC market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact the HER2+ NSCLC market growth.
| HER2+ Non-Small Cell Lung Cancer Report Metrics | Details |
| Study Period | 2020–2034 |
| HER2+ Non-Small Cell Lung Cancer Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| HER2 NSCLC (Mutant, Overexpression, and Amplification) Market Size in 2023 | USD 850 Million |
| HER2+ Non-Small Cell Lung Cancer Market CAGR | 13.2% |
| Key HER2+ Non-Small Cell Lung Cancer Companies | Jiangsu HengRui Medicine, Boehringer Ingelheim, Bayer, Forward Pharmaceuticals, ORIC Pharmaceuticals, Iambic Therapeutics, ArriVent BioPharma, Mersana Therapeutics, GSK, Nuvalent, and others |
| Key HER2+ Non-Small Cell Lung Cancer Therapies | Pyrotinib, Zongertinib, BAY2927088, FWD1509, ORIC-114, IAM1363, Firmonertinib, XMT-2056, NVL-330, and others |
Scope of the HER2+ Non-Small Cell Lung Cancer Market Report
- HER2+ Non-Small Cell Lung Cancer Therapeutic Assessment: HER2+ Non-Small Cell Lung Cancer current marketed and emerging therapies
- HER2+ Non-Small Cell Lung Cancer Market Dynamics: Conjoint Analysis of Emerging HER2+ Non-Small Cell Lung Cancer Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, HER2+ Non-Small Cell Lung Cancer Market Access and Reimbursement
Discover more about HER2+ NSCLC drugs in development @ HER2+ Non-Small Cell Lung Cancer Clinical Trials
Table of Contents
| 1 | Key Insights |
| 2 | Report Introduction |
| 3 | Executive Summary |
| 4 | Key Events |
| 5 | Epidemiology and Market Forecast Methodology |
| 6 | HER2 NSCLC Market Overview at a Glance |
| 6.1 | Market Share (%) Distribution of HER2 NSCLC by LoT in 2020 in the 7MM |
| 6.2 | Market Share (%) Distribution of HER2 NSCLC by LoT in 2034 in the 7MM |
| 6.3 | Market Share (%) Distribution of HER2 NSCLC by Therapies in 2020 in the 7MM |
| 6.4 | Market Share (%) Distribution of HER2 NSCLC by Therapies in 2034 in the 7MM |
| 7 | Disease Background and Overview |
| 7.1 | Introduction |
| 7.2 | HER2 NSCLC Alternations |
| 7.3 | Biological Consequences of HER2 Alterations |
| 7.3.1 | Signs and Symptoms of NSCLC |
| 7.3.2 | Risk Factors of Lung Cancer |
| 7.4 | Diagnosis of HER2 NSCLC |
| 7.4.1 | Stages of NSCLC |
| 7.4.2 | Staging System |
| 7.5 | Current Treatment Practices: HER2 NSCLC |
| 8 | Guidelines |
| 8.1 | NCCN Guideline for HER2 NSCLC (2024) |
| 8.2 | ESMO Guideline for HER2 NSCLC (2022) |
| 8.3 | ASCO Guideline for HER2 NSCLC (2024) |
| 8.4 | The Japan Lung Cancer Society Guidelines for HER2 NSCLC (2023) |
| 8.5 | National Consensus of the Spanish Society of Pathology and Spanish Society of Medical Oncology Guidelines for HER2 NSCLC (2023) |
| 9 | Epidemiology and Patient Population of HER2 NSCLC in the 7MM |
| 9.1 | Key Findings |
| 9.2 | Assumptions and Rationales |
| 9.3 | Total Incident Cases of NSCLC in the 7MM |
| 9.4 | The United States |
| 9.4.1 | Total Incident Cases of NSCLC in the United States |
| 9.4.2 | Gender-specific Cases of NSCLC in the United States |
| 9.4.3 | Age-specific Cases of NSCLC in the United States |
| 9.4.4 | Total Incident Cases of NSCLC by Histology in the United States |
| 9.4.5 | Total Incident Cases of NSCLC by Stage in the United States |
| 9.4.6 | Total Incident Cases of Advanced HER2 NSCLC in the United States |
| 9.5 | EU4 and the UK |
| 9.5.1 | Total Incident Cases of NSCLC in EU4 and the UK |
| 9.5.2 | Gender-specific Cases of NSCLC in EU4 and the UK |
| 9.5.3 | Age-specific Cases of NSCLC in EU4 and the UK |
| 9.5.4 | Total Incident Cases of NSCLC by Histology in EU4 and the UK |
| 9.5.5 | Total Incident Cases of NSCLC by Stage in EU4 and the UK |
| 9.5.6 | Total Incident Cases of Advanced HER2 NSCLC in EU4 and the UK |
| 9.6 | Japan |
| 9.6.1 | Total Incident Cases of NSCLC in Japan |
| 9.6.2 | Gender-specific Cases of NSCLC in Japan |
| 9.6.3 | Age-specific Cases of NSCLC in Japan |
| 9.6.4 | Total Incident Cases of NSCLC by Histology in Japan |
| 9.6.5 | Total Incident Cases of NSCLC by Stage in Japan |
| 9.6.6 | Total Incident Cases of Advanced HER2 NSCLC in Japan |
| 10 | Patient Journey |
| 11 | Marketed Drugs |
| 11.1 | Key Cross Competition |
| 11.2 | ENHERTU (trastuzumab deruxtecan): Daiichi Sankyo and AstraZeneca |
| 11.2.1 | Product Description |
| 11.2.2 | Regulatory Milestones |
| 11.2.3 | Other Development Activities |
| 11.2.4 | Clinical Development |
| 11.2.4.1 | Clinical Trial Information |
| 11.2.5 | Safety and Efficacy |
| 12 | Emerging Drugs |
| 12.1 | Key Cross Competition |
| 12.2 | Pyrotinib: Jiangsu HengRui Medicine |
| 12.2.1 | Product Description |
| 12.2.2 | Other Development Activities |
| 12.2.3 | Clinical Development |
| 12.2.3.1 | Clinical Trial Information |
| 12.2.4 | Safety and Efficacy |
| 12.3 | BAY 2927088: Bayer |
| 12.3.1 | Product Description |
| 12.3.2 | Other Development Activities |
| 12.3.3 | Clinical Development |
| 12.3.3.1 | Clinical Trial Information |
| 12.3.4 | Safety and Efficacy |
| 13 | HER2 NSCLC: 7MM Analysis |
| 13.1 | Key Findings |
| 13.2 | Market Outlook |
| 13.3 | Key Market Forecast Assumptions |
| 13.4 | Conjoint Analysis |
| 13.4.1 | Cost Assumptions and Rebates |
| 13.4.2 | Pricing Trends |
| 13.4.3 | Analogue Assessment |
| 13.4.4 | Launch Year and Therapy Uptakes |
| 13.5 | Total Market Size of HER2 NSCLC in the 7MM |
| 13.6 | Market Size of HER2 NSCLC by Therapies in the 7MM |
| 13.7 | United States Market Size |
| 13.7.1 | Total Market Size of HER2 NSCLC in the United States |
| 13.7.2 | Market Size of HER2 NSCLC by Therapies in the United States |
| 13.8 | EU4 and the UK Market Size |
| 13.8.1 | Total Market Size of HER2 NSCLC in EU4 and the UK |
| 13.8.2 | Market Size of HER2 NSCLC by Therapies in EU4 and the UK |
| 13.9 | Japan Market Size |
| 13.9.1 | Total Market Size of HER2 NSCLC in Japan |
| 13.9.2 | Market Size of HER2 NSCLC by Therapies in Japan |
| 14 | Unmet Needs |
| 15 | SWOT Analysis |
| 16 | KOL Views |
| 17 | Market Access and Reimbursement |
| 17.1 | United States |
| 17.2 | EU4 and the UK |
| 17.2.1 | Germany |
| 17.2.2 | France |
| 17.2.3 | Italy |
| 17.2.4 | Spain |
| 17.2.5 | United Kingdom |
| 17.3 | Japan |
| 17.4 | Market Access and Reimbursement of HER2 NSCLC |
| 18 | Appendix |
| 18.1 | Bibliography |
| 18.2 | Acronyms and Abbreviations |
| 18.3 | Report Methodology |
| 19 | DelveInsight Capabilities |
| 20 | Disclaimer |
| 21 | About DelveInsight |
Related Reports
HER2+ Non-Small Cell Lung Cancer Epidemiology Forecast
HER2+ Non-Small Cell Lung Cancer Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted HER2+ NSCLC epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
HER2+ Market Size, Target Population, Competitive Landscape & Market Forecast – 2034 report deliver an in-depth understanding of the inhibtors, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key HER2+ companies including Zymeworks, Jazz Pharmaceuticals, Ambrx, AnBogen Therapeutics, Enliven Therapeutics, Roche, among others.
Non-Small Cell Lung Cancer Market
Non-Small Cell Lung Cancer Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key NSCLC companies, including EMD Serono, Merck, Cellular Biomedicine Group, Inc., Celgene, CellSight Technologies, Inc., BeyondSpring Pharmaceuticals Inc., J Ints Bio, Forward Pharmaceuticals Co., Ltd., AstraZeneca, Bristol-Myers Squibb, Teligene US, Rain Oncology Inc, ReHeva Biosciences, Inc., Amgen, Novartis, RedCloud Bio, Parexel, Vitrac Therapeutics, LLC, Mythic Therapeutics, Instil Bio, Mirati Therapeutics Inc., Daiichi Sankyo, Inc., AstraZeneca, Precision Biologics, Inc, Promontory Therapeutics Inc., Palobiofarma SL, Regeneron Pharmaceuticals, Revolution Medicines, Inc., Cullinan Oncology, LLC, Iovance Biotherapeutics, Inc., Innate Pharma, among others.
Non-small Cell Lung Cancer Pipeline
Non-small Cell Lung Cancer Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key non-small cell lung cancer companies, including BridgeBio Pharma, Daiichi Sankyo, EMD Serono, Merck, BridgeBio Pharma, Abbvie, Pfizer, Eli Lilly and Company BioNTech SE, Shenzhen TargetRx, Taiho Pharmaceutical, Chong Kun Dang, Bristol Myers Squibb, Innovent Biologics, Xuanzhu Biopharmaceutical, Bayer, GeneScience Pharmaceuticals, InventisBio, Apollomics, Imugene, Ono Pharmaceutical, Pierre Fabre, Jiangsu Hengrui Medicine Co., Bristol-Myers Squibb, Surface Oncology, Inhibrx, Sinocelltech, Mirati Therapeutics, REVOLUTION Medicines, Yong Shun Technology Development, Iovance Biotherapeutics, Galecto Biotech, among others.
HER2-mutant Non-Small Cell Lung Cancer Pipeline
HER2-mutant Non-Small Cell Lung Cancer Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key HER2-mutant non-small cell lung cancer companies, including Dizal Pharmaceuticals, Puma Biotechnology, AstraZeneca, Jiangsu Hengrui Medicine, among others.
Oncology Conference Coverage Services
DelveInsight’s Oncology Conference Coverage Services offer a thorough analysis of outcomes from major events like ASCO, ESMO, ASH, AACR, ASTRO, SOHO, SITC, the European CAR T-cell Meeting, and IASLC. This detailed examination provides businesses with essential insights for competitive intelligence and market trend forecasting, supporting the formulation of future strategies.
Get in touch with us today to learn how we can provide AACR coverage exclusively for you at the AACR Meeting 2025
Other Business Consulting Services
Healthcare Competitive Intelligence
Healthcare Portfolio Management
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
Distribution channels: Healthcare & Pharmaceuticals Industry, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

